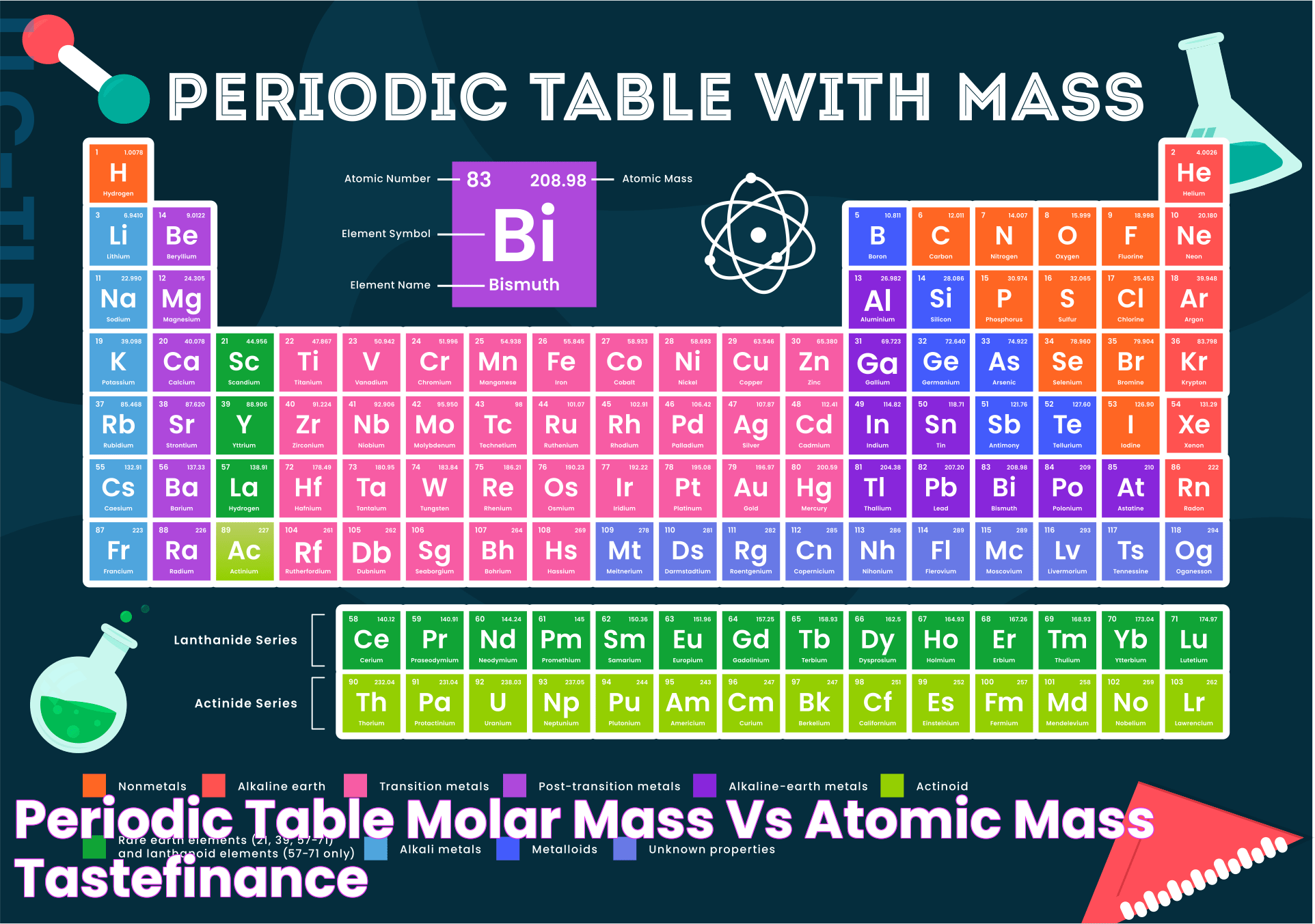
When delving into the realms of chemistry, two terms that often surface are "atomic mass" and "molar mass." While they may seem similar to the untrained eye, they hold distinct differences and are used in various contexts within the scientific field. Understanding these differences is fundamental for anyone venturing into the study of chemistry, as it lays the groundwork for comprehending more complex concepts. This article will elucidate the differences and applications of atomic mass vs molar mass, providing you with a clear understanding of their roles in scientific calculations.
Atomic mass refers to the mass of a single atom, typically measured in atomic mass units (amu), reflecting the mass of protons and neutrons in the nucleus. It's a fundamental property that helps scientists understand elements' characteristics and behavior in nature. On the other hand, molar mass pertains to the mass of one mole of a substance, expressed in grams per mole (g/mol), which is crucial for stoichiometric calculations in chemistry. Both these measurements are pivotal in different branches of chemistry and are used to perform calculations that range from simple to complex.
In this article, we will explore the intricacies of atomic mass vs molar mass, highlighting their definitions, differences, and applications in various scientific scenarios. By the end of this reading, you will be equipped with the knowledge to differentiate between these two terms, understand their significance in chemical equations, and apply them accurately in laboratory experiments and real-world applications. Let's embark on this educational journey to demystify these essential components of chemistry.
Read also:Deltas Buddy Tickets A Guide To Maximizing Your Travel Experience
Table of Contents
- Definition of Atomic Mass
- Definition of Molar Mass
- How is Atomic Mass Calculated?
- How is Molar Mass Calculated?
- Atomic Mass vs Molar Mass: What are the Differences?
- Importance of Atomic Mass in Chemistry
- Importance of Molar Mass in Chemistry
- Applications of Atomic Mass
- Applications of Molar Mass
- Atomic Mass in the Periodic Table
- Molar Mass in Chemical Reactions
- How Does Atomic Mass Affect Material Properties?
- How Does Molar Mass Affect Chemical Equilibria?
- Atomic Mass and Isotopes
- Molar Mass and Compound Purity
- Frequently Asked Questions
- Conclusion
Definition of Atomic Mass
Atomic mass is a fundamental concept in chemistry, representing the mass of an individual atom. It is most commonly expressed in atomic mass units (amu), a unit of mass used to express atomic and molecular weights. The atomic mass of an element is approximately equivalent to the number of protons and neutrons in the atom, as electrons have negligible mass. The atomic mass is often found on the periodic table and is used to identify elements and their isotopes.
Atomic mass is crucial in understanding the properties of elements and their behavior in chemical reactions. It serves as a basic identifier for elements, allowing scientists to differentiate between isotopes and predict how an element may react with others. For scientists and researchers, atomic mass provides a foundation for more complex chemical calculations and experimentation.
Additionally, atomic mass plays a crucial role when examining isotopes. Isotopes are atoms of the same element with different numbers of neutrons, leading to different atomic masses. These variations in atomic mass can significantly impact the stability and radioactive properties of isotopes, making atomic mass an essential tool for nuclear chemistry.
Definition of Molar Mass
Molar mass is a key concept in chemistry, defined as the mass of one mole of a substance, usually expressed in grams per mole (g/mol). It serves as a bridge between the microscopic world of atoms and molecules and the macroscopic world of grams and liters. Molar mass is essential for converting between the number of moles of a substance and its mass, facilitating stoichiometric calculations in chemical reactions.
The molar mass of a compound is calculated by summing the atomic masses of all the atoms in its chemical formula. For example, the molar mass of water (H₂O) is the sum of the molar masses of two hydrogen atoms (2 x 1.01 g/mol) and one oxygen atom (16.00 g/mol), resulting in a molar mass of 18.02 g/mol. This calculation is fundamental for chemists to determine the quantities of reactants and products in a chemical reaction.
Molar mass is also integral to understanding the properties of compounds. It allows scientists to calculate concentrations, determine reaction yields, and predict how a substance will behave under different conditions. By comprehending molar mass, chemists can perform precise measurements and calculations, ensuring accurate and reproducible results in their experiments.
Read also:Meet Taylor Zakhar Perez A Rising Star In Hollywood
How is Atomic Mass Calculated?
Calculating atomic mass involves considering the isotopic composition of an element. The atomic mass of an element is determined by averaging the masses of its isotopes, weighted by their natural abundance. This weighted average provides a value that reflects the relative abundance of each isotope in a natural sample of the element.
For instance, consider the element chlorine, which has two stable isotopes: chlorine-35 and chlorine-37. Chlorine-35 has an atomic mass of approximately 34.97 amu and constitutes about 75% of natural chlorine, while chlorine-37 has an atomic mass of approximately 36.97 amu and makes up about 25%. The atomic mass of chlorine is calculated as follows:
- Chlorine-35: 34.97 amu x 0.75 = 26.23 amu
- Chlorine-37: 36.97 amu x 0.25 = 9.24 amu
Adding these values gives an average atomic mass of approximately 35.47 amu for chlorine.
Understanding atomic mass calculations is vital for accurately identifying elements and predicting their behavior in chemical reactions. By considering isotopic composition, scientists can precisely determine the atomic mass, enabling more accurate calculations and predictions in various scientific fields.
How is Molar Mass Calculated?
Calculating molar mass involves summing the atomic masses of all atoms in a compound's molecular formula. This process provides the molar mass, which serves as a conversion factor between moles and grams, facilitating stoichiometric calculations in chemical reactions.
To calculate molar mass, follow these steps:
- Identify the molecular formula of the compound.
- Determine the atomic mass of each element in the formula from the periodic table.
- Multiply the atomic mass of each element by the number of atoms of that element in the formula.
- Add the resulting values to obtain the molar mass of the compound.
For example, let's calculate the molar mass of sulfuric acid (H₂SO₄):
- Hydrogen (H): 2 x 1.01 g/mol = 2.02 g/mol
- Sulfur (S): 1 x 32.07 g/mol = 32.07 g/mol
- Oxygen (O): 4 x 16.00 g/mol = 64.00 g/mol
The molar mass of sulfuric acid is the sum of these values: 2.02 + 32.07 + 64.00 = 98.09 g/mol.
Understanding how to calculate molar mass is essential for performing accurate stoichiometric calculations, determining reaction yields, and predicting compound properties. Mastering this skill allows chemists to conduct precise experiments and obtain reliable results.
Atomic Mass vs Molar Mass: What are the Differences?
While atomic mass and molar mass are related concepts, they differ in several key aspects. Understanding these differences is essential for accurately performing calculations and interpreting results in chemistry.
Definition: Atomic mass refers to the mass of a single atom, expressed in atomic mass units (amu), and considers the isotopic composition of the element. Molar mass, on the other hand, is the mass of one mole of a substance, expressed in grams per mole (g/mol), and is calculated by summing the atomic masses of all atoms in the compound's molecular formula.
Units: Atomic mass is measured in atomic mass units (amu), a unit of mass specifically for atomic-scale measurements. Molar mass is measured in grams per mole (g/mol), a unit that bridges the microscopic and macroscopic worlds.
Application: Atomic mass is used to identify elements and isotopes, predict their behavior in chemical reactions, and understand the properties of materials. Molar mass is used in stoichiometric calculations to determine the quantities of reactants and products, calculate concentrations, and predict compound behavior under different conditions.
Calculation: Atomic mass is calculated by averaging the isotopic masses of an element, weighted by their natural abundance. Molar mass is calculated by summing the atomic masses of all atoms in a compound's molecular formula.
Understanding these differences allows chemists to accurately perform calculations and interpret experimental results, ensuring successful scientific investigation and discovery.
Importance of Atomic Mass in Chemistry
Atomic mass plays a fundamental role in chemistry, serving as a crucial parameter for understanding elements' properties and behavior. It is indispensable for identifying elements and isotopes, predicting chemical reactions, and comprehending material properties.
One of the primary uses of atomic mass is in identifying elements and their isotopes. By determining the atomic mass, scientists can distinguish between different isotopes of the same element, which can have varying properties and applications. For example, isotopes of carbon, such as carbon-12 and carbon-14, have different atomic masses and are used in diverse scientific fields, including radiocarbon dating and nuclear medicine.
Atomic mass is also vital for predicting chemical reactions. It helps scientists understand how elements will interact with each other, enabling them to predict the products of chemical reactions and design new compounds with specific properties. Additionally, atomic mass allows for precise calculations of reaction yields, ensuring accurate and reproducible results in experimental chemistry.
Furthermore, atomic mass is integral to understanding material properties. It influences the density, melting point, and boiling point of materials, affecting their usability in various applications. By comprehending atomic mass, scientists can develop new materials with tailored properties, advancing technology and innovation.
Importance of Molar Mass in Chemistry
Molar mass is a vital concept in chemistry, essential for stoichiometric calculations, determining reaction yields, and comprehending compound properties. It serves as a conversion factor between moles and grams, facilitating precise measurements and calculations in chemical reactions.
One of the primary uses of molar mass is in stoichiometry, the quantitative study of reactants and products in chemical reactions. By knowing the molar mass of a compound, chemists can calculate the number of moles in a given mass, enabling them to determine the quantities of reactants and products needed for a reaction. This calculation ensures accurate and reproducible results, allowing scientists to design efficient and effective experiments.
Molar mass is also crucial for determining reaction yields. By calculating the molar masses of reactants and products, chemists can predict the amount of product formed in a reaction and assess the efficiency of the reaction. This information is vital for optimizing chemical processes and developing new compounds with desired properties.
Furthermore, molar mass is integral to understanding compound properties. It influences the solubility, vapor pressure, and boiling point of compounds, affecting their behavior under different conditions. By comprehending molar mass, scientists can predict how compounds will behave in various environments, enabling them to design new materials and applications.
Applications of Atomic Mass
Atomic mass finds numerous applications in various scientific fields, from identifying elements and isotopes to predicting chemical reactions and understanding material properties. Its versatility makes it an indispensable tool for chemists, physicists, and other scientists.
One of the primary applications of atomic mass is in identifying elements and their isotopes. By determining the atomic mass, scientists can distinguish between different isotopes of the same element, each with unique properties and applications. This information is crucial for fields such as radiocarbon dating, nuclear medicine, and environmental science.
Atomic mass is also used in predicting chemical reactions. It helps scientists understand how elements will interact with each other, enabling them to predict the products of chemical reactions and design new compounds with specific properties. This application is vital for developing new materials and advancing technology.
Furthermore, atomic mass is integral to understanding material properties. It influences the density, melting point, and boiling point of materials, affecting their usability in various applications. By comprehending atomic mass, scientists can develop new materials with tailored properties, advancing technology and innovation.
Applications of Molar Mass
Molar mass plays a crucial role in chemistry, with applications ranging from stoichiometric calculations to understanding compound properties and predicting reaction yields. Its importance makes it an essential tool for chemists and other scientists.
One of the primary applications of molar mass is in stoichiometry, the quantitative study of reactants and products in chemical reactions. By knowing the molar mass of a compound, chemists can calculate the number of moles in a given mass, enabling them to determine the quantities of reactants and products needed for a reaction. This calculation ensures accurate and reproducible results, allowing scientists to design efficient and effective experiments.
Molar mass is also crucial for determining reaction yields. By calculating the molar masses of reactants and products, chemists can predict the amount of product formed in a reaction and assess the efficiency of the reaction. This information is vital for optimizing chemical processes and developing new compounds with desired properties.
Furthermore, molar mass is integral to understanding compound properties. It influences the solubility, vapor pressure, and boiling point of compounds, affecting their behavior under different conditions. By comprehending molar mass, scientists can predict how compounds will behave in various environments, enabling them to design new materials and applications.
Atomic Mass in the Periodic Table
The periodic table is a fundamental tool in chemistry, providing essential information about the elements and their properties. Atomic mass is one of the key parameters displayed on the periodic table, serving as a crucial identifier for elements and their isotopes.
Atomic mass is usually listed below the element's symbol on the periodic table. It reflects the average atomic mass of the element, considering its isotopic composition and natural abundance. This information is vital for identifying elements and distinguishing between isotopes, each with unique properties and applications.
The periodic table organizes elements by increasing atomic number, allowing scientists to predict trends in atomic mass and other properties. For example, atomic mass generally increases as you move from left to right and top to bottom on the periodic table. This trend is due to the addition of protons and neutrons in the nucleus, resulting in heavier elements.
Understanding atomic mass in the context of the periodic table is crucial for identifying elements, predicting their behavior in chemical reactions, and understanding material properties. By comprehending this information, scientists can perform accurate calculations and make informed decisions in experimental chemistry.
Molar Mass in Chemical Reactions
Molar mass is a critical parameter in chemical reactions, serving as a conversion factor between moles and grams and facilitating stoichiometric calculations. Its importance makes it an essential tool for chemists and other scientists.
One of the primary applications of molar mass in chemical reactions is in stoichiometry, the quantitative study of reactants and products. By knowing the molar mass of a compound, chemists can calculate the number of moles in a given mass, enabling them to determine the quantities of reactants and products needed for a reaction. This calculation ensures accurate and reproducible results, allowing scientists to design efficient and effective experiments.
Molar mass is also crucial for determining reaction yields. By calculating the molar masses of reactants and products, chemists can predict the amount of product formed in a reaction and assess the efficiency of the reaction. This information is vital for optimizing chemical processes and developing new compounds with desired properties.
Furthermore, molar mass is integral to understanding the properties of compounds in chemical reactions. It influences the solubility, vapor pressure, and boiling point of compounds, affecting their behavior under different conditions. By comprehending molar mass, scientists can predict how compounds will behave in various environments, enabling them to design new materials and applications.
How Does Atomic Mass Affect Material Properties?
Atomic mass plays a crucial role in determining the properties of materials, influencing their density, melting point, boiling point, and other characteristics. Understanding how atomic mass affects material properties is essential for scientists and engineers in developing new materials and applications.
One of the primary ways atomic mass affects material properties is through density. The mass of an atom influences the density of a material, affecting its weight and buoyancy. Heavier atoms result in denser materials, which can have a significant impact on their usability in various applications.
Atomic mass also influences the melting point and boiling point of materials. Heavier atoms typically require more energy to break the bonds holding them together, resulting in higher melting and boiling points. This property is vital for understanding the thermal stability of materials and their behavior under different conditions.
Furthermore, atomic mass can affect the mechanical properties of materials, such as hardness, strength, and elasticity. By understanding the relationship between atomic mass and material properties, scientists can design new materials with tailored characteristics, advancing technology and innovation.
How Does Molar Mass Affect Chemical Equilibria?
Molar mass plays a significant role in chemical equilibria, influencing the concentrations of reactants and products and determining the equilibrium position. Understanding how molar mass affects chemical equilibria is essential for chemists and other scientists in predicting reaction behavior and optimizing chemical processes.
One of the primary ways molar mass affects chemical equilibria is through concentration calculations. The molar mass of a compound determines the number of moles in a given mass, affecting the concentration of reactants and products in a reaction. This information is vital for predicting the equilibrium position and understanding how changes in concentration will shift the equilibrium.
Molar mass also influences the rate of reaction, which can affect the equilibrium position. Heavier compounds may react more slowly, impacting the concentrations of reactants and products and altering the equilibrium position. By understanding the relationship between molar mass and reaction rate, scientists can predict how changes in reactant or product concentrations will affect the equilibrium.
Furthermore, molar mass can affect the solubility of compounds, influencing the concentrations of reactants and products in solution. By comprehending how molar mass affects solubility, scientists can predict how changes in concentration will shift the equilibrium, enabling them to design efficient chemical processes and develop new compounds.
Atomic Mass and Isotopes
Isotopes are atoms of the same element that have different numbers of neutrons, resulting in different atomic masses. Understanding the relationship between atomic mass and isotopes is essential for scientists in fields such as nuclear chemistry, environmental science, and medicine.
The atomic mass of an element is determined by averaging the masses of its isotopes, weighted by their natural abundance. This weighted average provides a value that reflects the relative abundance of each isotope in a natural sample of the element. By determining the atomic mass, scientists can distinguish between different isotopes and predict their behavior in chemical reactions.
Isotopes have unique properties and applications, making them valuable tools in various scientific fields. For example, isotopes of carbon, such as carbon-12 and carbon-14, are used in radiocarbon dating and nuclear medicine, respectively. Understanding the relationship between atomic mass and isotopes is crucial for accurately identifying isotopes and predicting their behavior in chemical reactions.
Furthermore, isotopes can have significant impacts on the stability and radioactive properties of elements. By comprehending the relationship between atomic mass and isotopes, scientists can predict the stability and behavior of isotopes, enabling them to design new materials and applications.
Molar Mass and Compound Purity
Molar mass is a critical parameter for determining compound purity, influencing the accuracy and reliability of chemical measurements and calculations. Understanding the relationship between molar mass and compound purity is essential for chemists and other scientists in performing accurate experiments and developing new materials.
One of the primary ways molar mass affects compound purity is through stoichiometric calculations. The molar mass of a compound determines the number of moles in a given mass, affecting the accuracy of stoichiometric calculations and the determination of reaction yields. By understanding the relationship between molar mass and compound purity, scientists can perform accurate measurements and calculations, ensuring reliable results.
Molar mass also influences the solubility and stability of compounds, affecting their purity and behavior under different conditions. By comprehending how molar mass affects solubility and stability, scientists can predict how compounds will behave in various environments, enabling them to design new materials and applications with desired properties.
Furthermore, molar mass can affect the accuracy of spectroscopic measurements, influencing the determination of compound purity. By understanding the relationship between molar mass and spectroscopic measurements, scientists can perform accurate analyses and ensure reliable results in their experiments.
Frequently Asked Questions
What is atomic mass?
Atomic mass is the mass of a single atom, expressed in atomic mass units (amu). It considers the isotopic composition of the element and is used to identify elements and their isotopes.
What is molar mass?
Molar mass is the mass of one mole of a substance, expressed in grams per mole (g/mol). It is used in stoichiometric calculations to determine the quantities of reactants and products in chemical reactions.
How are atomic mass and molar mass different?
Atomic mass refers to the mass of a single atom, while molar mass refers to the mass of one mole of a substance. Atomic mass is expressed in atomic mass units (amu), while molar mass is expressed in grams per mole (g/mol).
How is atomic mass calculated?
Atomic mass is calculated by averaging the masses of an element's isotopes, weighted by their natural abundance. This weighted average provides a value that reflects the relative abundance of each isotope in a natural sample of the element.
How is molar mass calculated?
Molar mass is calculated by summing the atomic masses of all atoms in a compound's molecular formula. This calculation provides the molar mass, which serves as a conversion factor between moles and grams.
Why is understanding atomic mass and molar mass important?
Understanding atomic mass and molar mass is essential for performing accurate calculations and interpreting results in chemistry. They are critical for identifying elements and isotopes, predicting chemical reactions, and understanding compound properties.
Conclusion
In the journey through the intricacies of atomic mass vs molar mass, we've uncovered the essential roles these concepts play in the world of chemistry. From understanding the basic definitions to their applications in stoichiometric calculations and material sciences, both atomic mass and molar mass serve as foundational elements in scientific discovery and innovation. By grasping these concepts, scientists and students alike can accurately perform calculations, predict chemical reactions, and explore new frontiers in material development. As we continue to advance in technology and science, the importance of these fundamental concepts will remain pivotal, guiding researchers in the quest for knowledge and progress.