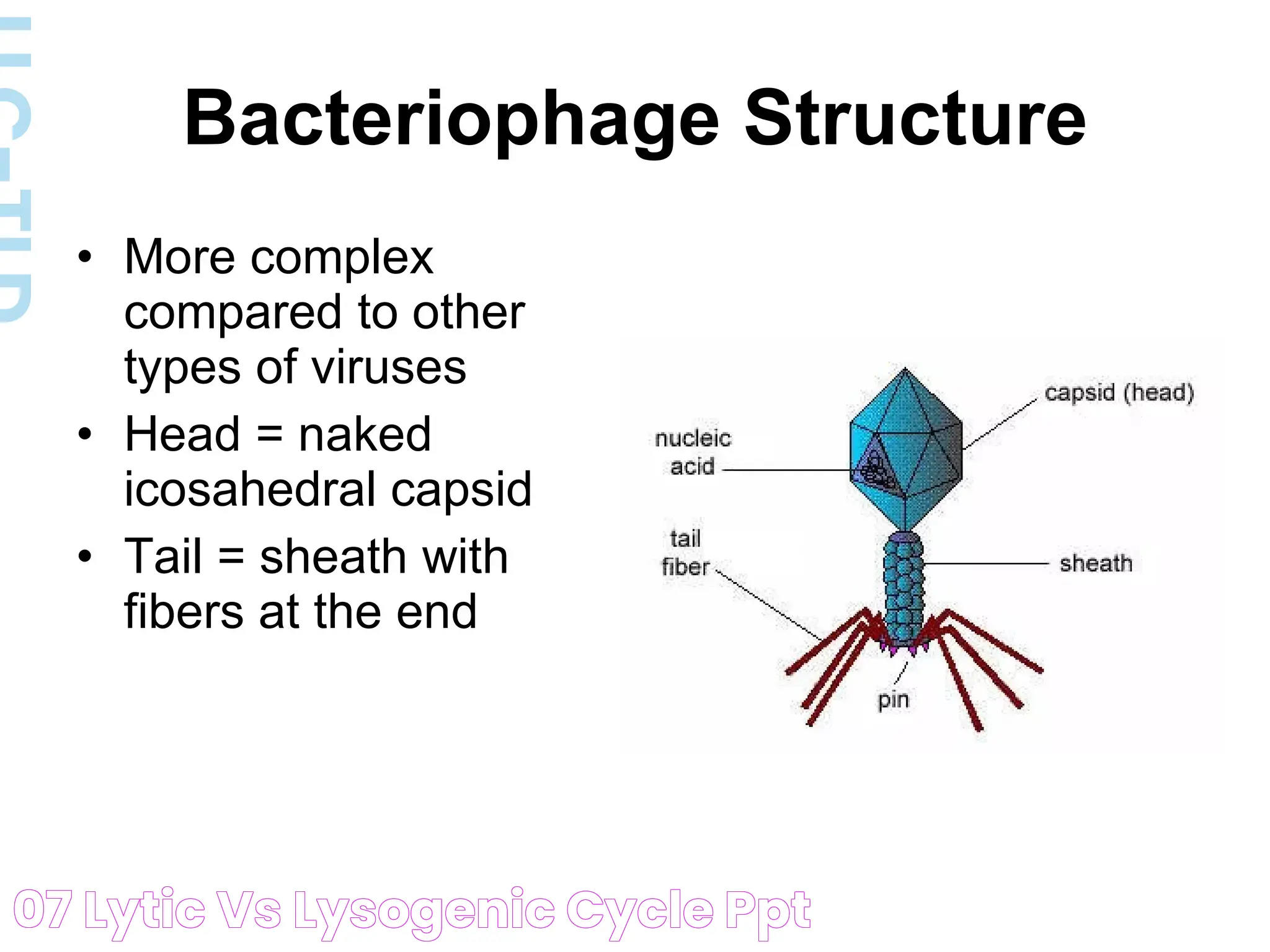
The interplay between viruses and their host cells is a fascinating arena of biological science, with the lytic cycle and lysogenic cycle standing at the forefront of viral replication strategies. Both cycles represent distinct pathways through which viruses propagate, albeit with different outcomes for the host cells. Understanding these processes is crucial for insights into viral behavior, disease propagation, and potential therapeutic interventions. This article delves into the intricacies of the lytic and lysogenic cycles, highlighting their differences, similarities, and broader implications in virology.
Viruses, while often perceived as mere agents of disease, are complex entities that have evolved sophisticated methods to hijack host cellular machinery for replication. The lytic cycle is characterized by rapid viral replication leading to cell lysis, whereas the lysogenic cycle involves the integration of viral DNA into the host genome, allowing the virus to persist in a dormant state. These cycles are not only pivotal to the life cycles of viruses but also play a significant role in shaping the genetic landscape of host organisms.
As we explore the contrasting dynamics of the lytic cycle vs lysogenic cycle, we will uncover how these processes impact viral evolution, influence genetic diversity, and contribute to the development of viral diseases. Additionally, this examination provides a foundation for understanding the potential for viral manipulation in gene therapy and vaccine development, showcasing the dual nature of viruses as both pathogens and tools for scientific advancement.
Read also:Top Spots For Haircuts Near You Find Your Perfect Style
Table of Contents
- Biological Foundations of Viral Cycles
- What is the Lytic Cycle?
- How Does the Lytic Cycle Operate?
- What is the Lysogenic Cycle?
- How Does the Lysogenic Cycle Function?
- Lytic Cycle vs Lysogenic: Key Differences
- Impact on Host Cells: Lytic and Lysogenic Cycles
- Role in Viral Evolution
- Genetic Diversity and Viruses
- Disease Propagation and Viral Cycles
- Therapeutic Implications
- Gene Therapy and Viral Vectors
- Vaccines and Viruses: Leveraging Viral Cycles
- FAQs
- Conclusion
Biological Foundations of Viral Cycles
Viruses are unique entities that straddle the line between life and non-life. Unlike living organisms, they cannot replicate independently and must hijack the cellular machinery of a host to propagate. This reliance on host cells is a defining feature of viral biology and underpins the mechanisms of the lytic and lysogenic cycles.
The lytic cycle is a classic representation of viral replication, where the virus invades the host cell, commandeers its machinery to produce new viral particles, and eventually causes the cell to burst, releasing the progeny virions. This cycle is rapid, aggressive, and often results in the destruction of the host cell, making it a primary pathway for acute viral infections.
In contrast, the lysogenic cycle represents a more subtle and long-term strategy. Here, viral DNA integrates into the host genome, where it can remain dormant for extended periods. This allows the virus to persist without causing immediate harm to the host, and under certain conditions, the virus may switch to the lytic cycle, initiating active replication.
What is the Lytic Cycle?
The lytic cycle is a well-studied viral replication process characterized by the production of new virions within a host cell, culminating in cell lysis. This cycle is utilized by many bacteriophages and some animal viruses, providing a rapid means of viral propagation.
Key Steps in the Lytic Cycle
- Attachment: The virus attaches to specific receptors on the surface of the host cell, ensuring specificity.
- Penetration: Viral genetic material is injected into the host cell, leaving the viral coat outside.
- Biosynthesis: The host's cellular machinery is hijacked to replicate viral DNA and synthesize viral proteins.
- Assembly: New viral particles are assembled from the replicated genetic material and proteins.
- Lysis: The host cell bursts, releasing newly formed virions to infect other cells.
This cycle is efficient in generating large numbers of viral progeny, but it often results in the death of the host cell, limiting the virus's ability to maintain a long-term presence in the host population.
How Does the Lytic Cycle Operate?
The operation of the lytic cycle hinges on the virus's ability to effectively commandeer the host's cellular machinery. Upon attachment, the virus injects its genetic material, which may be either DNA or RNA, into the host cell. This genetic material then takes over the host's transcription and translation processes to produce viral components.
Read also:Gta 6 Cost Everything You Need To Know
Mechanisms of Host Cell Manipulation
Viruses have evolved various strategies to manipulate host cells:
- **Inhibition of Host Defense Mechanisms:** Viruses may produce proteins that inhibit host immune responses, allowing them to replicate unhindered.
- **Redirection of Cellular Resources:** Viral proteins can redirect cellular resources towards viral replication, depriving the host of essential functions.
- **Genomic Integration:** Some viruses integrate their genome into the host DNA, ensuring persistence and transmission to progeny cells.
The culmination of these processes is the assembly of new virions and the eventual lysis of the host cell, releasing the viral progeny to continue the cycle.
What is the Lysogenic Cycle?
The lysogenic cycle represents a more insidious form of viral replication. Unlike the lytic cycle, where viral replication results in the destruction of the host cell, the lysogenic cycle allows the virus to integrate its genetic material into the host genome, where it can remain dormant for extended periods.
Integration and Dormancy
The hallmark of the lysogenic cycle is the integration of viral DNA into the host's genetic material. This integrated viral DNA, known as a prophage in bacteriophages, is replicated along with the host's DNA during cell division, allowing the virus to persist across generations of host cells.
During periods of dormancy, the virus may not produce new virions, effectively hiding from the host's immune system. However, environmental triggers or stressors can induce the prophage to excise from the host genome, entering the lytic cycle and initiating active replication.
How Does the Lysogenic Cycle Function?
The lysogenic cycle begins with the attachment and penetration of the viral genome into the host cell, similar to the lytic cycle. However, instead of hijacking the host's machinery for immediate replication, the viral genome integrates into the host DNA.
Mechanisms of Viral Integration
Integration is facilitated by viral enzymes known as integrases, which catalyze the insertion of viral DNA into the host genome. This process is highly specific, often targeting conserved regions of the host DNA to ensure stable integration.
Once integrated, the prophage remains dormant, coexisting with the host genome. This dormancy can be maintained indefinitely, with the viral DNA being passively replicated during host cell division. However, under certain conditions, the prophage may be induced to enter the lytic cycle, leading to active viral replication and cell lysis.
Lytic Cycle vs Lysogenic: Key Differences
The lytic and lysogenic cycles represent two distinct viral replication strategies, each with unique characteristics and implications for viral propagation and host interaction.
Comparative Analysis
- Replication Speed: The lytic cycle is rapid, resulting in the quick production of new virions, while the lysogenic cycle is slower, with replication occurring over extended periods.
- Impact on Host Cells: The lytic cycle leads to cell lysis and death, whereas the lysogenic cycle allows the host cell to survive, often without immediate harm.
- Genomic Integration: Only the lysogenic cycle involves the integration of viral DNA into the host genome, allowing for latent infection and transmission across generations.
- Viral Persistence: The lysogenic cycle enables long-term viral persistence, while the lytic cycle is more transient, with viruses needing to continuously infect new cells.
These differences highlight the adaptability of viruses and their ability to exploit different replication strategies to maximize survival and propagation.
Impact on Host Cells: Lytic and Lysogenic Cycles
The impact of viral replication on host cells is profound, influencing cellular function, survival, and genetic integrity. The lytic cycle, with its destructive nature, often results in significant cellular damage, while the lysogenic cycle can alter host genetics without immediate harm.
Cellular Consequences
The lytic cycle's most immediate impact is cell death, as the host cell is destroyed to release new virions. This can lead to tissue damage and inflammation, contributing to the symptoms of viral infections.
In contrast, the lysogenic cycle may not cause immediate harm, but the integration of viral DNA can have long-term consequences. The insertion of viral genes can disrupt normal cellular functions, potentially leading to oncogenesis or other genetic disorders. Additionally, the viral genome may be reactivated under certain conditions, leading to a switch to the lytic cycle and subsequent cell lysis.
Role in Viral Evolution
Viral replication cycles play a crucial role in viral evolution, influencing genetic diversity, adaptability, and the emergence of new viral strains. The lytic and lysogenic cycles contribute to this evolutionary process in different ways.
Mechanisms of Evolutionary Change
The lytic cycle, with its rapid replication, provides opportunities for genetic mutations and recombination events, leading to the emergence of new viral variants. This genetic diversity is essential for viral adaptation to changing environments and host defenses.
The lysogenic cycle, on the other hand, facilitates horizontal gene transfer, as the integration of viral DNA into host genomes can lead to the exchange of genetic material between viruses and hosts. This process can drive the evolution of both viruses and host organisms, influencing traits such as virulence and host range.
Genetic Diversity and Viruses
Genetic diversity is a hallmark of viral populations, enabling them to adapt to diverse environments and host immune responses. The lytic and lysogenic cycles contribute to this diversity through different mechanisms.
Sources of Viral Diversity
- Mutation: The rapid replication of the lytic cycle increases the likelihood of mutations, providing a rich source of genetic variation.
- Recombination: Viruses can exchange genetic material with one another or with host cells, leading to novel genotypes.
- Gene Transfer: The lysogenic cycle facilitates horizontal gene transfer, allowing viruses to acquire new genes from host genomes.
These mechanisms ensure that viral populations remain highly diverse, enhancing their ability to evade host defenses and exploit new ecological niches.
Disease Propagation and Viral Cycles
The replication strategies of viruses have significant implications for disease propagation. The lytic and lysogenic cycles influence how viral infections spread and persist within host populations.
Patterns of Infection
Viruses employing the lytic cycle often cause acute infections, characterized by rapid onset and severe symptoms. These infections can spread quickly through populations, leading to outbreaks and epidemics.
The lysogenic cycle, by contrast, is associated with latent infections, where the virus remains dormant for extended periods. These infections may not produce immediate symptoms but can reactivate under certain conditions, leading to recurrent or chronic disease.
Therapeutic Implications
The understanding of viral replication cycles offers valuable insights for the development of antiviral therapies. Targeting specific stages of the lytic or lysogenic cycles can provide effective means of controlling viral infections.
Strategies for Intervention
Antiviral drugs can be designed to target key steps in the lytic cycle, such as viral attachment, penetration, or replication. By inhibiting these processes, the spread of the virus can be curtailed.
For viruses utilizing the lysogenic cycle, strategies may focus on preventing viral integration or reactivation. Gene editing technologies, such as CRISPR-Cas, hold promise for excising viral DNA from host genomes, offering potential cures for latent infections.
Gene Therapy and Viral Vectors
Viruses, with their ability to deliver genetic material into host cells, have become valuable tools in gene therapy. The lysogenic cycle, in particular, offers a means of stable gene integration, essential for therapeutic applications.
Applications of Viral Vectors
Viral vectors derived from the lysogenic cycle can be engineered to carry therapeutic genes into target cells. This approach has been used to treat genetic disorders, such as cystic fibrosis and hemophilia, by correcting defective genes.
The use of viral vectors in gene therapy highlights the dual nature of viruses, as both pathogens and tools for medical advancement, underscoring the potential for harnessing their capabilities for human benefit.
Vaccines and Viruses: Leveraging Viral Cycles
Vaccines represent a powerful tool in combating viral infections, and understanding viral replication cycles is crucial for their development. Both the lytic and lysogenic cycles offer opportunities for vaccine design.
Vaccine Development Strategies
- Live Attenuated Vaccines: These vaccines use weakened viruses that replicate via the lytic cycle, stimulating a strong immune response without causing disease.
- Viral Vector Vaccines: Utilizing viruses from the lysogenic cycle as vectors, these vaccines deliver antigenic genes to host cells, eliciting immunity.
- DNA/RNA Vaccines: These vaccines leverage the replication mechanisms of viruses to produce viral proteins, training the immune system to recognize and combat infections.
The development of vaccines based on viral cycles showcases the innovative use of viral biology to enhance public health, demonstrating the potential for science to turn viral mechanisms to humanity's advantage.
FAQs
- What is the main difference between the lytic and lysogenic cycles? The lytic cycle results in the rapid destruction of the host cell, while the lysogenic cycle involves the integration of viral DNA into the host genome, allowing for long-term persistence without immediate harm.
- How do viruses decide between the lytic and lysogenic cycles? Environmental conditions, host cell type, and viral genetic factors can influence the decision to enter the lytic or lysogenic cycle, with some viruses capable of switching between the two.
- Can a virus be both lytic and lysogenic? Yes, many viruses can undergo both cycles, starting with lysogenic integration and later switching to lytic replication under specific triggers.
- What are the risks of lysogenic viruses? Lysogenic viruses can cause long-term genetic changes in host cells, potentially leading to cancer or other genetic disorders.
- How do antiviral drugs target the lytic cycle? Antiviral drugs can inhibit various stages of the lytic cycle, such as attachment, replication, or assembly, to prevent viral propagation.
- Are there any benefits to the lysogenic cycle? The lysogenic cycle can be harnessed for gene therapy and vaccine development, offering stable genetic integration for therapeutic purposes.
Conclusion
The study of viral replication cycles, particularly the lytic and lysogenic cycles, provides profound insights into the complex interactions between viruses and host cells. These cycles not only define the strategies employed by viruses to propagate and survive but also influence the evolutionary dynamics, genetic diversity, and disease propagation within host populations.
Understanding the nuances of the lytic cycle vs lysogenic cycle is crucial for developing effective antiviral strategies, advancing gene therapy, and creating innovative vaccines. As we continue to explore the intricacies of viral biology, the potential to harness these mechanisms for scientific and medical advancements remains vast, offering hope for combating viral diseases and improving human health.
For more in-depth information on viral replication mechanisms, readers can refer to scholarly articles and textbooks on virology, or visit ScienceDirect for a comprehensive overview of viral life cycles.