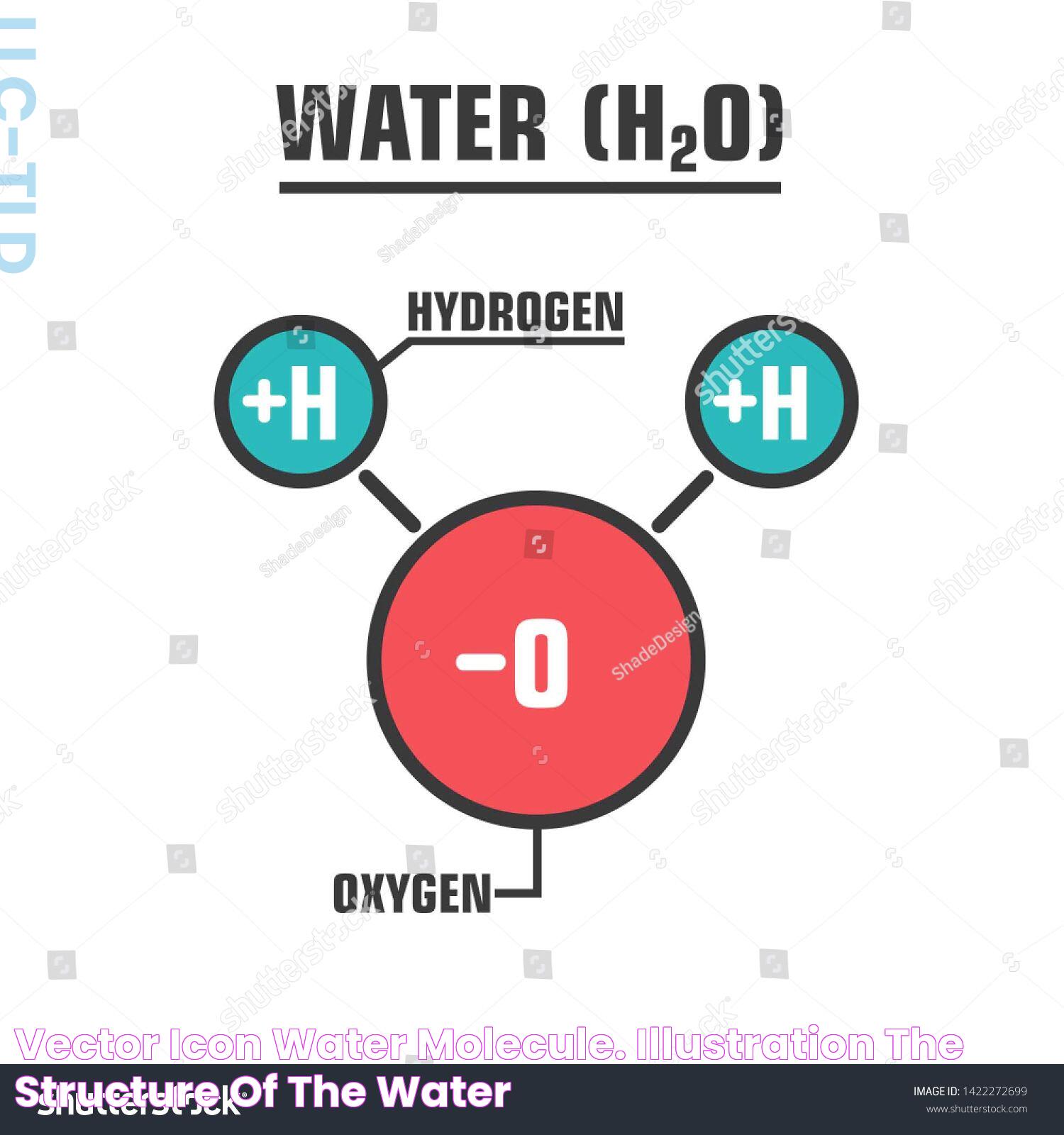
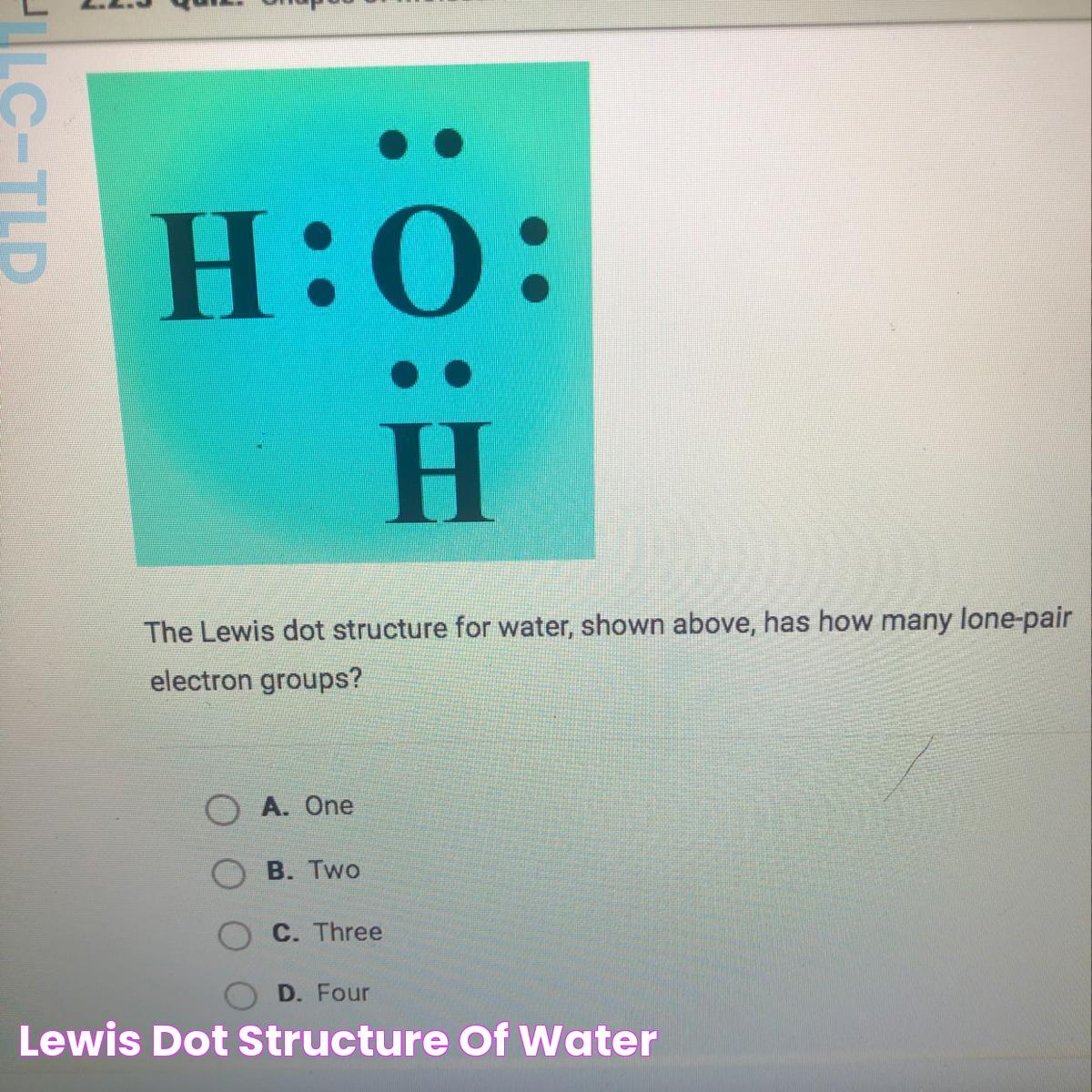
The Lewis structure for water is a fundamental concept in chemistry that plays a pivotal role in understanding molecular bonding. By visualizing the specific arrangement of atoms within a molecule, Lewis structures allow us to depict the distribution of electrons around atoms. This is crucial for predicting molecular shapes, reactivity, and properties. Water, being one of the most vital compounds on Earth, serves as an excellent example for studying Lewis structures due to its simplicity and significance.
Water, with its chemical formula H₂O, is composed of two hydrogen atoms and one oxygen atom. The Lewis structure for water not only helps us understand the bonding between these atoms but also provides insight into the molecule's polarity, hydrogen bonding, and many other unique properties. Knowing the Lewis structure aids in grasping how water interacts with other substances, forming the basis for countless chemical reactions and biological processes.
Understanding the Lewis structure for water is not merely an academic exercise but a necessity for anyone delving into chemistry, biology, or environmental science. The depiction of water's molecular structure opens the door to discovering its behavior in different states and environments, which is essential for fields ranging from biochemistry to climate science. This guide will walk you through the steps of drawing the Lewis structure for water, delving into its nuances, and exploring its implications in various scientific domains.
Read also:Warhammer 40k Khorne The Chaos God Of Blood And Battle
Table of Contents
- What is the Lewis Structure?
- Importance of Lewis Structure for Water
- Steps to Draw the Lewis Structure for Water
- Common Mistakes in Drawing Lewis Structures
- Why Does the Lewis Structure Matter?
- How Does Lewis Structure Affect Water Properties?
- Lewis Structure and Polarity
- Bonding and Angles in Water Molecule
- The Role of Electron Pairs
- Applications of Water Lewis Structure
- How to Teach Lewis Structure to Beginners?
- What Are the Limitations of Lewis Structures?
- Frequently Asked Questions
- External Resources
- Conclusion
What is the Lewis Structure?
The Lewis structure is a graphical representation of the valence electrons in a molecule. Named after Gilbert N. Lewis, who introduced the concept in 1916, these structures illustrate the bonding between atoms and the lone pairs of electrons that may exist. Lewis structures are essential for predicting the geometry and reactivity of molecules.
In a Lewis structure, the chemical symbols represent atoms, and lines or pairs of dots represent bonds and lone pairs. Electrons are depicted as dots placed around the atomic symbols, showing how they are shared or paired. By illustrating the valence electrons, Lewis structures help chemists visualize how atoms are connected in a molecule, providing insights into molecular stability and reactions.
Understanding the Lewis structure for water involves recognizing how hydrogen and oxygen share electrons to form stable bonds. The oxygen atom, with six valence electrons, forms two single bonds with hydrogen atoms, each sharing one electron to complete the valence shell. This arrangement, depicted in the Lewis structure, is fundamental to understanding water's chemical nature.
Importance of Lewis Structure for Water
The Lewis structure for water is not just a theoretical concept but a practical tool in various scientific fields. It is crucial for understanding water's unique properties, including its polarity, hydrogen bonding, and high specific heat capacity. These properties are integral to water's role as a universal solvent and its behavior in the environment.
In biology, the Lewis structure for water explains how it interacts with biomolecules, influencing protein folding, enzyme activity, and cellular processes. In chemistry, it aids in predicting water's reactivity and interactions with other compounds. Environmental scientists use it to model water's role in climate systems and ecosystems.
By mastering the Lewis structure for water, students and professionals can gain insights into the molecular mechanisms underlying these phenomena. This understanding is fundamental to advancing research and developing technologies in fields ranging from medicine to environmental science.
Read also:Stamp Costs Demystified Pricing History And More
Steps to Draw the Lewis Structure for Water
Drawing the Lewis structure for water involves a systematic approach to represent the distribution of electrons. Here are the steps to create an accurate depiction:
- Count the total valence electrons: Oxygen has six valence electrons, and each hydrogen has one, totaling eight electrons.
- Arrange the atoms: Place oxygen in the center and hydrogen atoms on either side, as oxygen is more electronegative.
- Form bonds: Draw single bonds between oxygen and each hydrogen, using two electrons for each bond.
- Distribute remaining electrons: Place the remaining four electrons as lone pairs around oxygen to complete its octet.
- Check for stability: Ensure each atom has a complete valence shell: hydrogen with two electrons and oxygen with eight.
These steps help visualize the covalent bonds and lone pairs in a water molecule, providing a clearer understanding of its structure and properties.
Common Mistakes in Drawing Lewis Structures
Even though drawing Lewis structures may seem straightforward, common mistakes can lead to inaccurate representations. Here are some pitfalls to avoid:
- Miscounting valence electrons: Ensure accurate counting of electrons for each atom to avoid errors in bonding.
- Incorrect atom placement: Place more electronegative atoms centrally, especially in polyatomic molecules.
- Overlooking lone pairs: Remember to include unshared electrons, which affect molecular geometry and reactivity.
- Inaccurate bonding: Double-check the number of bonds, especially in molecules with resonance structures.
- Neglecting formal charges: Ensure the formal charge is minimized for the most stable structure.
By being mindful of these mistakes, students and professionals can create accurate and reliable Lewis structures, enhancing their understanding of molecular chemistry.
Why Does the Lewis Structure Matter?
The Lewis structure is a cornerstone of molecular chemistry, offering a visual representation of how atoms bond and interact. It matters because it provides insights into:
- Molecular Geometry: Understanding the arrangement of atoms helps predict shapes and angles, crucial for reactivity and properties.
- Chemical Bonding: Visualizing covalent and ionic bonds aids in predicting chemical behavior and stability.
- Reactivity: The distribution of electrons impacts how molecules interact and react with others.
- Polarity: Determining electron distribution helps assess molecular polarity, essential for understanding solubility and interactions.
Grasping the Lewis structure for water and other molecules provides foundational knowledge for studying complex chemical systems and reactions.
How Does Lewis Structure Affect Water Properties?
The Lewis structure for water is intricately linked to its unique properties, which are essential for life and environmental processes. These properties include:
- Polarity: The bent shape and uneven electron distribution create a polar molecule, leading to strong hydrogen bonding.
- Solvent Capabilities: Water's polarity allows it to dissolve ionic and polar substances, making it a versatile solvent.
- High Specific Heat: Hydrogen bonding leads to high heat capacity, moderating temperature fluctuations in nature.
- Cohesion and Adhesion: Water molecules stick together and to surfaces, supporting capillary action and transpiration.
Understanding these properties through the Lewis structure provides insights into water's vital role in biological, chemical, and environmental systems.
Lewis Structure and Polarity
The Lewis structure for water reveals its polar nature, which is fundamental to its behavior and interactions. The key aspects include:
- Electron Distribution: Oxygen's higher electronegativity attracts electrons, creating a partial negative charge.
- Molecular Shape: The bent shape, with an angle of about 104.5°, results in a dipole moment.
- Hydrogen Bonding: The polar nature facilitates hydrogen bonds, influencing properties like boiling point and solubility.
Water's polarity, highlighted by its Lewis structure, is central to its role as a solvent and its interactions in biological systems.
Bonding and Angles in Water Molecule
The Lewis structure for water helps elucidate the bonding and geometric angles within the molecule, key to its properties. The essential points include:
- Covalent Bonds: Single covalent bonds form between oxygen and each hydrogen, sharing electrons.
- Bond Angle: The H-O-H angle is approximately 104.5°, influenced by lone pair repulsion.
- Lone Pairs: Two lone pairs on oxygen contribute to the bent shape and electron distribution.
Understanding these aspects through the Lewis structure helps predict water's behavior in various chemical and physical contexts.
The Role of Electron Pairs
Electron pairs, both shared and unshared, play a critical role in the Lewis structure for water, influencing its shape and reactivity. Key elements include:
- Bonding Pairs: Shared electrons form covalent bonds, stabilizing the molecule.
- Lone Pairs: Unshared pairs on oxygen affect geometry and polarity.
- Electron Repulsion: Lone pair repulsion causes the bent shape and influences molecular interactions.
Recognizing the impact of electron pairs in the Lewis structure for water enhances understanding of its chemical behavior and properties.
Applications of Water Lewis Structure
The Lewis structure for water has wide-ranging applications in science and industry, influencing research and development in various fields. Notable applications include:
- Environmental Science: Modeling water's role in climate systems and pollution control.
- Biochemistry: Understanding water's interactions with biomolecules and cellular processes.
- Industrial Chemistry: Predicting water's behavior in reactions and processes, such as catalysis and solvent systems.
The insights gained from the Lewis structure for water are crucial for advancing knowledge and technology across diverse disciplines.
How to Teach Lewis Structure to Beginners?
Teaching the Lewis structure for water to beginners requires a clear and engaging approach. Here are some strategies:
- Use Visual Aids: Diagrams and models help visualize electron distribution and bonding.
- Interactive Exercises: Hands-on activities and drawing exercises reinforce concepts.
- Real-World Examples: Relating structures to everyday substances makes learning relevant.
- Step-by-Step Guidance: Break down the process of drawing structures into manageable steps.
By employing these techniques, educators can effectively convey the importance and applications of the Lewis structure for water, fostering a deeper understanding among students.
What Are the Limitations of Lewis Structures?
While the Lewis structure for water is a valuable tool, it has limitations that must be recognized. These include:
- Simplistic Representation: Lewis structures may not fully capture complex molecular shapes or electron distributions.
- Resonance Structures: Some molecules require multiple structures to depict electron sharing accurately.
- Unrepresentative of Reality: They do not account for molecular dynamics or electronic delocalization.
Understanding these limitations helps chemists and students use Lewis structures effectively while recognizing the need for more advanced models in complex scenarios.
Frequently Asked Questions
What is the significance of the Lewis structure for water?
The Lewis structure for water is significant because it provides insights into water's molecular bonding, geometry, and properties, essential for understanding its role in various scientific and industrial contexts.
How does the Lewis structure illustrate water's polarity?
The Lewis structure shows the arrangement of electrons and the bent shape of the water molecule, highlighting its polar nature and the resulting hydrogen bonding capabilities.
Why is it important to learn the Lewis structure for water?
Learning the Lewis structure for water is crucial for understanding its chemical behavior, interactions with other substances, and its vital role in biological and environmental systems.
Can the Lewis structure for water be used to predict reactivity?
Yes, the Lewis structure helps predict water's reactivity by illustrating its electron distribution and bonding, which influence its interactions with other molecules.
What are some common mistakes in drawing Lewis structures?
Common mistakes include miscounting valence electrons, incorrect atom placement, overlooking lone pairs, inaccurate bonding, and neglecting formal charges.
How do electron pairs affect the water molecule's shape?
Electron pairs, especially lone pairs, create repulsion that influences the bent shape of the water molecule, affecting its geometry and properties.
External Resources
For further reading and exploration of Lewis structures and their applications, consider visiting the following resources:
- Chemguide: Lewis Structures - A comprehensive guide to understanding and drawing Lewis structures.
- PubChem: Water - Detailed information on water's chemical structure and properties.
Conclusion
The Lewis structure for water is an essential tool for understanding the molecular bonding, geometry, and properties of this vital compound. By mastering the depiction of water's electron distribution, students and professionals gain valuable insights into its behavior and interactions across various scientific and industrial fields. While recognizing its limitations, the Lewis structure remains a foundational concept that enhances our comprehension of chemical systems and their applications.