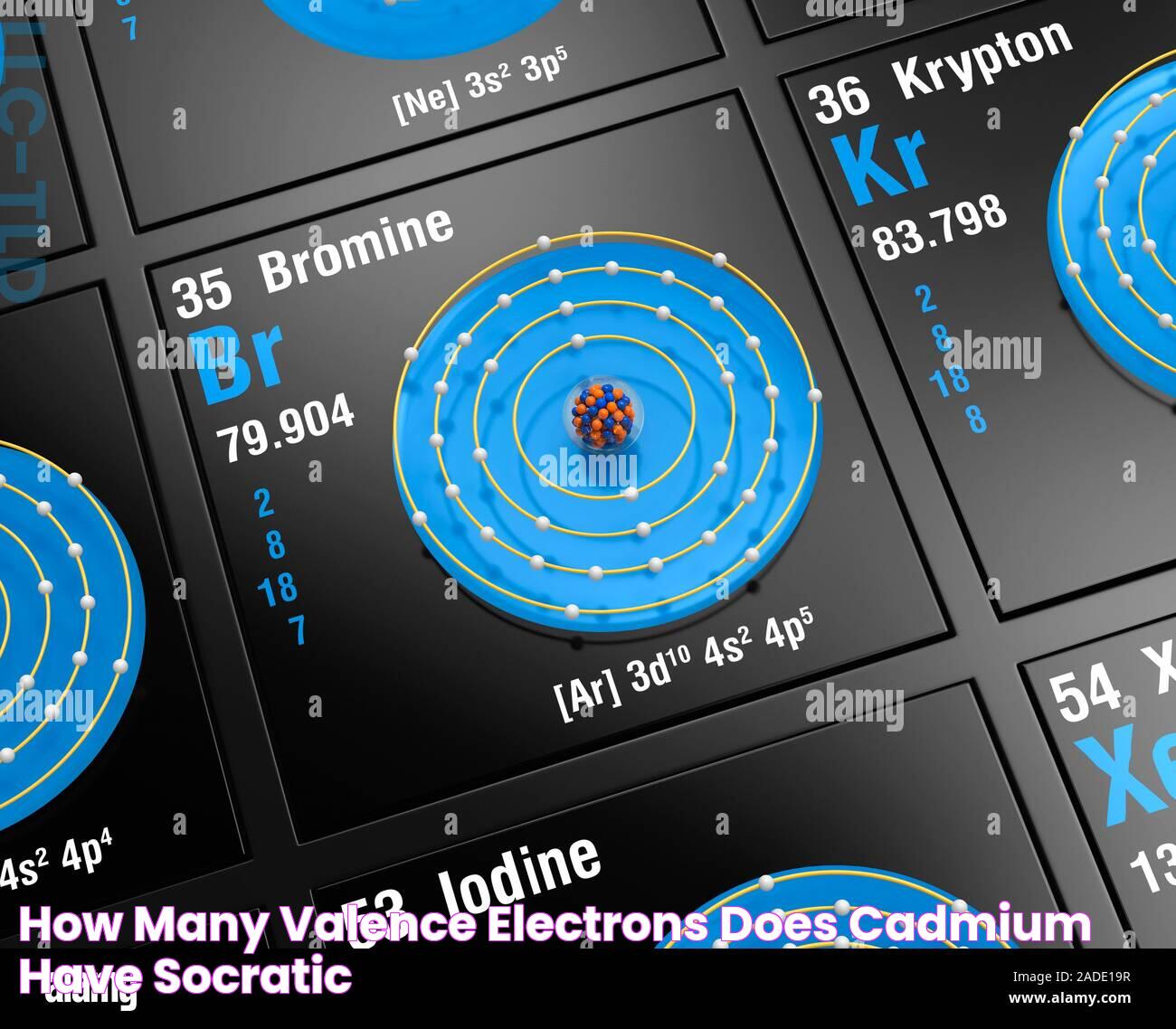
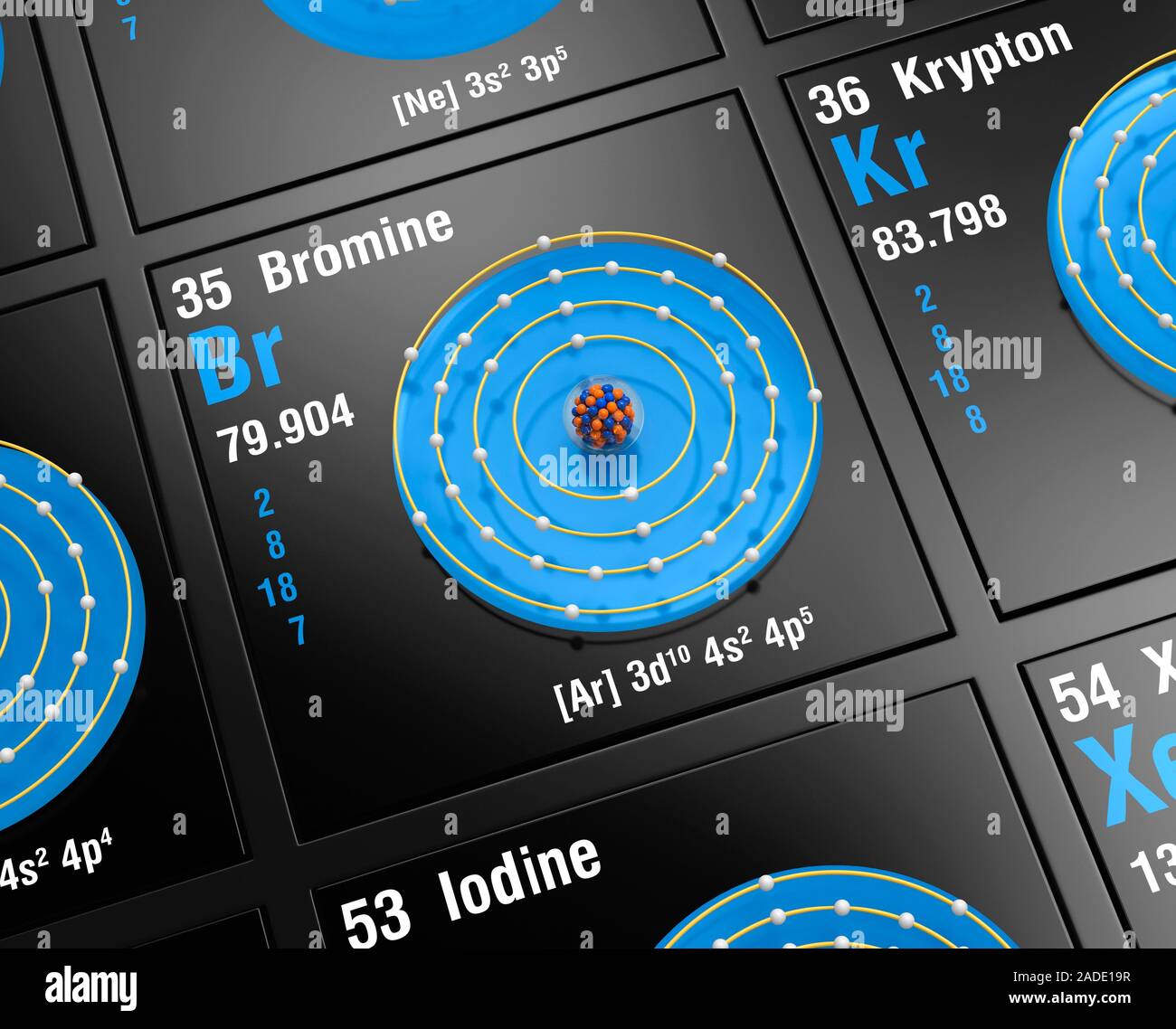
In the realm of chemistry, understanding the behavior of elements is crucial to grasping how they interact with each other. One such element is bromine, a member of the halogen group. Bromine's unique properties and its position in the periodic table make it an interesting subject of study, particularly when it comes to its valence electrons. Valence electrons are the outermost electrons of an atom and play a significant role in chemical bonding and reactions. For bromine, these electrons determine how it pairs with other elements, influencing its chemical properties and reactivity.
Bromine, with the chemical symbol Br, is known for its distinct reddish-brown color and is the only nonmetallic element that is liquid at room temperature. As a member of Group 17 in the periodic table, bromine shares characteristics with other halogens, such as chlorine and iodine. Its atomic number is 35, which means it has 35 protons in its nucleus. This also indicates that, in its neutral state, bromine has 35 electrons surrounding its nucleus. These electrons are arranged in shells around the nucleus, with the valence electrons being in the outermost shell. The configuration of these electrons is vital to understanding bromine's chemical behavior.
The study of valence electrons for bromine not only helps in understanding its reactivity but also sheds light on its role in various chemical processes. These electrons are responsible for the formation of bonds with other elements, which is essential in the formation of compounds. By examining the valence electrons of bromine, scientists and chemists can predict its behavior in reactions, its affinity for electrons, and its potential to form stable compounds. As we delve deeper into this topic, we will explore the significance of valence electrons, bromine's position in the periodic table, and its implications in chemical bonding.
Read also:Khal Drigo The Charismatic Leader And His Enduring Influence
Table of Contents
- What are Valence Electrons for Bromine?
- Why are Valence Electrons Important?
- Where is Bromine Located on the Periodic Table?
- How is the Valence Electron Configuration of Bromine Determined?
- What are the Chemical Properties of Bromine?
- What Compounds Does Bromine Form?
- How is Bromine Found in Nature?
- What are the Industrial Uses of Bromine?
- How Do Valence Electrons Affect Bonding?
- Is Bromine Safe for Health?
- How Do Bromine's Valence Electrons Compare to Other Halogens?
- What is the Environmental Impact of Bromine?
- What Experiments Involve Valence Electrons of Bromine?
- What are the Latest Research Advancements in Bromine Studies?
- Frequently Asked Questions
- Conclusion
What are Valence Electrons for Bromine?
Valence electrons are the outermost electrons of an atom and are crucial in determining how an element will react chemically. For bromine, these electrons reside in the fourth energy level, as bromine is located in the fourth period of the periodic table. Specifically, bromine has seven valence electrons in its outer shell. This is because bromine, with an atomic number of 35, has an electron configuration of [Ar] 3d10 4s2 4p5. The electrons in the 4p sublevel are considered the valence electrons.
The presence of seven valence electrons means that bromine needs only one more electron to achieve a stable octet configuration, similar to the noble gases. This makes bromine highly reactive, as it readily forms bonds with other elements to complete its outer shell. The tendency of bromine to gain an electron makes it a strong oxidizing agent, often forming negative ions in chemical reactions.
Bromine's valence electrons also play a pivotal role in its ability to form covalent bonds. In many compounds, bromine shares its valence electrons with other nonmetals, resulting in stable molecular structures. Understanding the behavior of these electrons is essential in predicting the types of bonds bromine will form and the nature of the compounds it will create.
Why are Valence Electrons Important?
Valence electrons are paramount in chemistry because they are the electrons involved in chemical bonding. These electrons determine the reactivity of an element, its ability to form bonds, and the type of bonds it forms. For elements like bromine, which are known for their reactivity, valence electrons are vital in understanding how the element interacts with others.
- Chemical Bonding: Valence electrons are involved in both ionic and covalent bonding. In ionic bonding, atoms gain or lose valence electrons to achieve a full outer shell, resulting in the formation of ions. In covalent bonding, atoms share valence electrons to attain stability.
- Reactivity: Elements with nearly filled or nearly empty valence shells (like bromine) are often highly reactive. Bromine's seven valence electrons mean it readily forms bonds to complete its outer shell.
- Periodic Trends: The number of valence electrons affects an element's position in the periodic table and its group characteristics. Bromine's valence electrons place it in Group 17, the halogens, known for their high reactivity.
Understanding valence electrons aids in predicting and explaining the behavior of elements in chemical reactions. For bromine, this understanding is crucial, given its role in forming various compounds and its widespread use in different industries.
Where is Bromine Located on the Periodic Table?
Bromine is located in Group 17 of the periodic table, which is also known as the halogen group. This group includes other elements like fluorine, chlorine, iodine, and astatine. All halogens have seven valence electrons, which contribute to their similar chemical behavior and high reactivity.
Read also:Remarkable Avalanche Goalies A Deep Dive Into Their Legacy
In terms of period, bromine is found in the fourth period of the periodic table. This period placement is significant because it indicates the number of electron shells an atom of bromine possesses. Bromine's electron configuration is [Ar] 3d10 4s2 4p5, with the 4p sublevel containing the valence electrons.
The position of bromine in the periodic table explains its properties and reactivity. As a halogen, bromine is highly electronegative, meaning it has a strong tendency to attract electrons to itself in a chemical bond. This property is due to its nearly complete valence shell, which drives its reactivity and ability to form compounds with a wide range of elements.
How is the Valence Electron Configuration of Bromine Determined?
Determining the valence electron configuration of bromine involves understanding its overall electron configuration and identifying the electrons in its outermost shell. Bromine's atomic number is 35, meaning it has 35 electrons in its neutral state. These electrons are distributed among different energy levels or shells.
The electron configuration of bromine is [Ar] 3d10 4s2 4p5. This notation indicates that bromine's electrons fill the 1s, 2s, 2p, 3s, 3p, 3d, 4s, and finally the 4p sublevels. The valence electrons are those in the 4s and 4p sublevels, totaling seven electrons. These are the electrons involved in chemical bonding and determine bromine's reactivity.
- Step 1: Identify the atomic number of bromine, which is 35.
- Step 2: Write the electron configuration using the Aufbau principle, filling the lower energy levels first.
- Step 3: Determine the valence electrons, which are the electrons in the outermost shell (4s and 4p).
This understanding of bromine's valence electron configuration is crucial for predicting its chemical behavior and the types of bonds it can form with other elements.
What are the Chemical Properties of Bromine?
Bromine is a highly reactive element with distinct chemical properties that define its interactions with other elements. As a member of the halogen group, bromine exhibits several characteristic behaviors due to its valence electrons.
- Reactivity: Bromine is highly reactive and readily forms compounds with a variety of elements. Its seven valence electrons make it eager to gain one more electron to achieve a stable octet configuration.
- Oxidizing Agent: Due to its high electronegativity, bromine acts as a strong oxidizing agent. It can accept electrons from other elements, leading to the formation of bromide ions (Br-).
- Halogen Characteristics: Like other halogens, bromine forms diatomic molecules (Br2) and participates in halogen exchange reactions. It can displace less reactive halogens from compounds.
- Bond Formation: Bromine forms both ionic and covalent bonds, depending on the elements it interacts with. In covalent bonds, bromine shares its valence electrons with nonmetals to form stable molecules.
Understanding these chemical properties is essential for utilizing bromine in various applications, from industrial processes to the synthesis of pharmaceuticals and agrochemicals.
What Compounds Does Bromine Form?
Bromine forms a wide range of compounds, thanks to its reactive nature and valence electrons. These compounds can be classified into several categories based on the types of bonds bromine forms with other elements.
- Ionic Compounds: Bromine forms ionic compounds with metals by gaining an electron to become a bromide ion (Br-). These compounds include sodium bromide (NaBr) and potassium bromide (KBr).
- Covalent Compounds: Bromine forms covalent bonds with nonmetals, sharing its valence electrons. Notable examples include bromine trifluoride (BrF3) and dibromomethane (CH2Br2).
- Organobromine Compounds: Bromine is involved in the formation of organobromine compounds, where it bonds with carbon atoms. These compounds are used in flame retardants, pharmaceuticals, and agrochemicals.
The diversity of bromine compounds highlights its versatility and importance in various chemical processes and industrial applications. Its ability to form stable bonds with a range of elements makes it a valuable component in many chemical reactions.
How is Bromine Found in Nature?
Bromine is not found in its elemental form in nature due to its high reactivity. Instead, it exists in the form of bromide salts in the Earth's crust and seawater. The majority of bromine is extracted from natural brine pools and salt lakes, where it is found in higher concentrations.
The process of extracting bromine from natural sources involves several steps:
- Collection: Bromine-rich brine is collected from natural sources such as salt lakes and underground brine wells.
- Oxidation: The bromide ions in the brine are oxidized to elemental bromine using chlorine gas.
- Distillation: The bromine, being volatile, is distilled from the solution and collected as a reddish-brown liquid.
Bromine's natural occurrence and extraction methods are significant for industrial production, as they provide a steady supply of this essential element for various applications.
What are the Industrial Uses of Bromine?
Bromine's unique properties and reactivity make it valuable for a range of industrial applications. Its uses span from flame retardants to pharmaceuticals, highlighting its versatility and importance in modern industry.
- Flame Retardants: Bromine compounds are widely used as flame retardants in plastics, textiles, and electronic devices. They help prevent the spread of fire by interfering with the chemical reactions that occur during combustion.
- Water Treatment: Bromine is used in water treatment processes to disinfect and purify water. It is an effective alternative to chlorine in swimming pools and hot tubs due to its stability and reduced odor.
- Pharmaceuticals: Bromine is a key component in the synthesis of various pharmaceuticals. Its compounds are used as intermediates in the production of drugs that treat conditions such as epilepsy and hypertension.
- Photography: Silver bromide is used in photographic films and papers due to its light-sensitive properties. This application has been largely replaced by digital technology but remains important for certain traditional photography methods.
The industrial applications of bromine underscore its significance in modern technology and manufacturing processes. Its ability to form stable compounds with other elements makes it a crucial component in various industries.
How Do Valence Electrons Affect Bonding?
Valence electrons are the primary contributors to chemical bonding, determining how atoms interact and form compounds. For bromine, its seven valence electrons play a key role in its bonding behavior and chemical properties.
In chemical bonding, valence electrons can be shared, gained, or lost to achieve stability. The two main types of bonds that involve valence electrons are:
- Ionic Bonds: In ionic bonding, atoms gain or lose valence electrons to form ions, resulting in the formation of ionic compounds. Bromine, with its high electronegativity, tends to gain an electron to form bromide ions (Br-), which then bond with cations to create ionic compounds.
- Covalent Bonds: In covalent bonding, atoms share valence electrons to achieve a full outer shell. Bromine can form covalent bonds with other nonmetals, sharing its valence electrons to create stable molecules. This type of bonding is common in organic compounds and diatomic bromine (Br2).
The behavior of bromine's valence electrons in bonding is crucial for understanding its chemical interactions and the nature of the compounds it forms. This knowledge is essential for predicting the properties and reactivity of bromine in various chemical processes.
Is Bromine Safe for Health?
Bromine is an essential element in various industrial applications, but its safety for health depends on its concentration and form. At low levels, bromine and its compounds are generally safe, but high concentrations can pose health risks.
Exposure to bromine can occur through inhalation, ingestion, or skin contact. Inhalation of bromine vapor can irritate the respiratory system, while skin contact can cause burns or irritation. Ingestion of bromine compounds, especially in large amounts, can lead to poisoning and adverse health effects.
In industrial settings, safety measures such as proper ventilation, protective equipment, and handling protocols are essential to minimize exposure to bromine. For consumers, bromine compounds used in products like flame retardants and water treatment are typically present in safe concentrations.
Overall, while bromine is a valuable element in various applications, it is important to handle it with care and adhere to safety guidelines to prevent health risks.
How Do Bromine's Valence Electrons Compare to Other Halogens?
Bromine, like other halogens, has seven valence electrons, which contributes to its high reactivity and similar chemical behavior to other elements in the group. However, there are subtle differences in the properties and reactivity of halogens due to their differing atomic sizes and electronegativities.
- Fluorine: The smallest and most electronegative halogen, fluorine, has a stronger tendency to attract electrons compared to bromine. Its reactivity is higher, and it forms stronger bonds with other elements.
- Chlorine: Chlorine is more electronegative than bromine and is more reactive. However, both elements can form similar types of compounds, with chlorine often being used as a substitute for bromine in certain applications.
- Iodine: Iodine is less electronegative and reactive than bromine. It has larger atomic and ionic sizes, which affect its bonding and chemical properties.
The comparison of bromine's valence electrons with other halogens provides insight into the periodic trends and chemical behaviors of these elements. Understanding these differences is crucial for selecting the appropriate halogen for specific chemical reactions and applications.
What is the Environmental Impact of Bromine?
Bromine and its compounds have both beneficial and adverse environmental impacts, depending on their use and concentration. The production and use of bromine compounds, such as flame retardants and pesticides, can lead to environmental concerns if not managed properly.
- Ozone Depletion: Certain bromine compounds, such as halons and methyl bromide, have been linked to ozone depletion. These compounds release bromine atoms into the atmosphere, which catalyze the breakdown of ozone molecules.
- Marine Pollution: Bromine compounds used in industrial processes can enter marine environments, affecting aquatic life. Proper disposal and treatment of bromine-containing waste are essential to mitigate this impact.
- Soil and Water Contamination: The use of bromine-based pesticides can lead to soil and water contamination, affecting ecosystems and human health. Regulations and alternative pest control methods are important for reducing this risk.
Efforts to minimize the environmental impact of bromine involve the development of safer alternatives, sustainable production practices, and stringent regulations. These measures aim to balance the benefits of bromine use with environmental protection.
What Experiments Involve Valence Electrons of Bromine?
Experiments involving bromine's valence electrons are essential for understanding its chemical behavior and reactivity. These experiments can range from simple demonstrations to complex laboratory research.
- Electron Configuration Studies: Experiments that explore bromine's electron configuration help students and researchers understand how its valence electrons contribute to its chemical properties.
- Reactivity Experiments: Testing bromine's reactivity with various elements and compounds demonstrates its tendency to gain electrons and form stable bonds.
- Bonding Experiments: Investigating the types of bonds bromine forms, such as ionic and covalent bonds, provides insight into its interactions with other elements.
These experiments are crucial for both educational purposes and advanced research, as they help elucidate the role of valence electrons in bromine's chemical behavior.
What are the Latest Research Advancements in Bromine Studies?
Recent research advancements in bromine studies focus on improving its applications, understanding its environmental impact, and developing safer alternatives. Key areas of research include:
- Flame Retardant Development: Researchers are exploring new bromine-based flame retardants that are more effective and environmentally friendly, reducing the release of harmful byproducts.
- Environmental Impact Studies: Studies on the environmental impact of bromine compounds aim to identify and mitigate their effects on ecosystems and human health.
- Alternative Pesticides: Research into alternative bromine-based pesticides seeks to provide effective pest control solutions with minimal environmental impact.
These advancements highlight the importance of continued research in understanding bromine's role in various applications and finding sustainable solutions for its use.
Frequently Asked Questions
What is the role of valence electrons in chemical reactions?
Valence electrons are crucial in chemical reactions as they determine how atoms bond with each other. They are involved in the formation of both ionic and covalent bonds, influencing the reactivity and stability of compounds.
How does bromine's reactivity compare to other elements?
Bromine is highly reactive, especially among nonmetals, due to its seven valence electrons. It is more reactive than iodine but less reactive than fluorine and chlorine, which are also halogens.
Can bromine be used in water treatment?
Yes, bromine is used in water treatment as a disinfectant. It is an effective alternative to chlorine, particularly in swimming pools and hot tubs, due to its stability and reduced odor.
What safety precautions are necessary when handling bromine?
When handling bromine, it's important to use protective equipment, such as gloves and goggles, and ensure proper ventilation. Bromine should be stored in a cool, dry place, away from incompatible substances.
Are there any natural sources of bromine?
Bromine is naturally found in brine pools and salt lakes. It is extracted from these sources through oxidation and distillation processes to be used in various industrial applications.
What is the significance of bromine in flame retardants?
Bromine is significant in flame retardants because its compounds can inhibit the chemical reactions that occur during combustion, helping to prevent the spread of fire in materials such as plastics and textiles.
Conclusion
Understanding the valence electrons for bromine is essential for comprehending its chemical behavior, reactivity, and the types of compounds it forms. Bromine's position in the periodic table, as a halogen with seven valence electrons, dictates its interactions with other elements and its tendency to achieve a stable octet configuration. From industrial applications to environmental impact, bromine plays a significant role in various fields, underscoring the importance of continued research and sustainable practices. By exploring the intricacies of bromine's valence electrons, we gain valuable insights into its properties and its contributions to science and industry.